The U.S. Food and Drug Administration issued emergency use authorization (EUA) for two vaccines for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Date Authorized
December 11, 2020
Manufacturer
Pfizer, Inc
Age Group
16 +
Doses Needed
2 doses, 3 weeks apart
Date Authorized
December 18, 2020
Manufacturer
ModernaTX, Inc
Age Group
18 +
Doses Needed
2 doses, 1 month apart
Because the U.S. supply of COVID-19 vaccine is expected to be limited at first, the Advisory Committee on Immunization Practices (ACIP) issues guidance to CDC providing recommendations to federal, state, and local governments about who should be vaccinated first. According to the CDC, these following groups of people should be vaccinated:
Healthcare personnel and residents of long-term care facilities should be offered the first doses of COVID-19 vaccines (1a)
Although the CDC has issued these recommendations, each state has its own plan for deciding who will be vaccinated first and how it will be distributed. Contact your local health department for more information on COVID-19 vaccination distribution in your area.
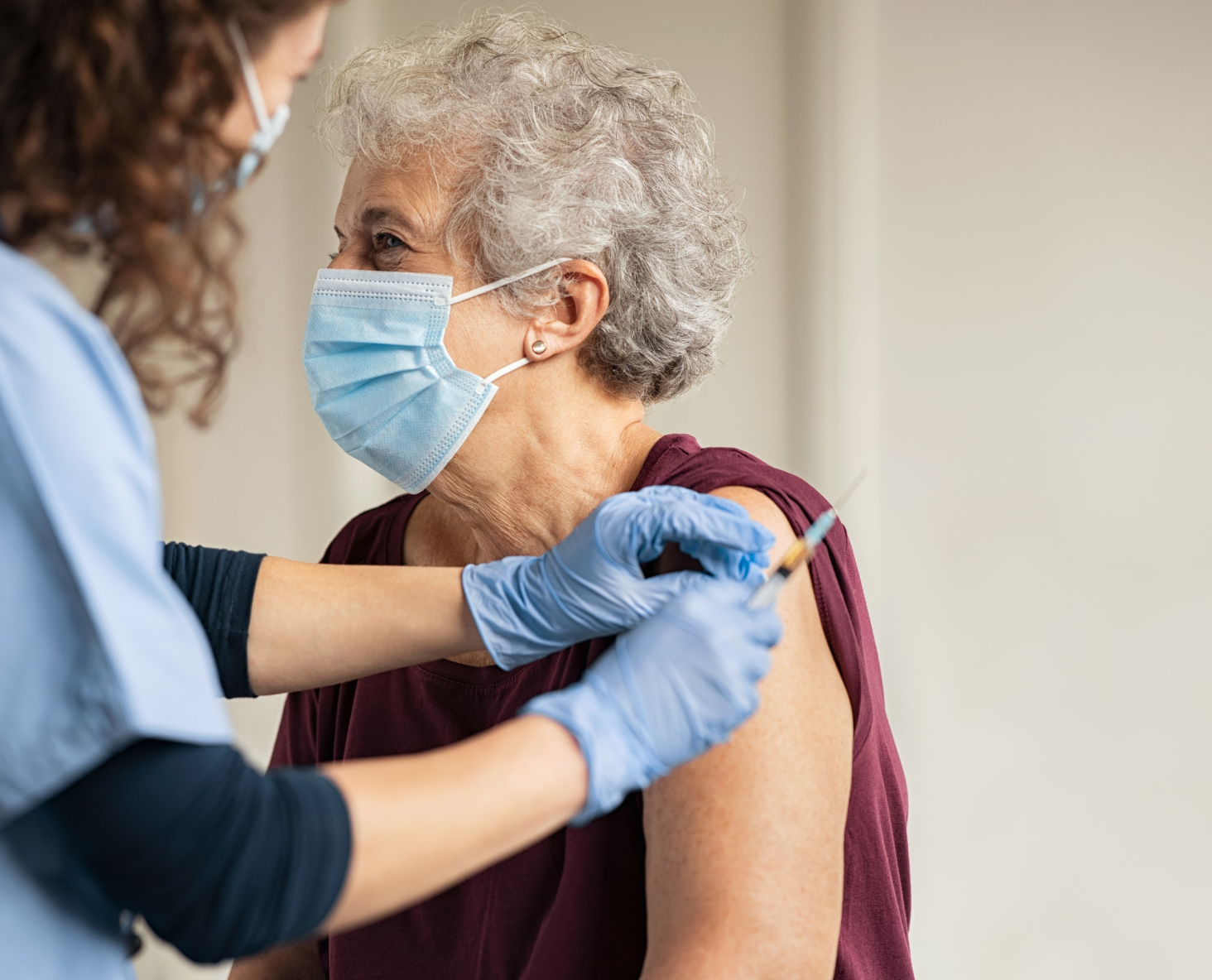
The AZOVA COVID-19 Vaccine Locator makes finding and booking your COVID-19 vaccine easy. Simply enter in your location to find vaccination providers near you and schedule a time for your vaccine.
Step 1
Search for a COVID-19 vaccination provider near you.
Step 2
Complete the assessment to determine vaccine eligibility.
Step 3
Arrive and receive your vaccine at the scheduled time.
Step 4
Receive your shareable COVID-19 vaccine record.
